Substitution At Vinylic Carbon

Journal of the american chemical society 2000 122 10 2294 2299.
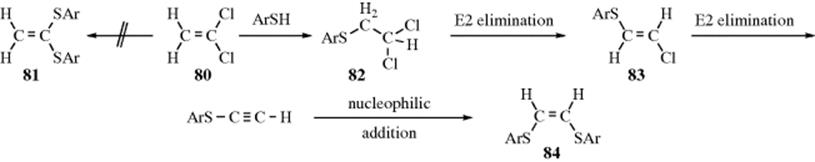
Substitution at vinylic carbon. Nucleophilic substitution at unactivated vinylic carbon. Since it involves a reaction of the nucleophile with the vinylic carbon atom it is also the one which in actual fact is most correctly described as a nucleophilic vinylic substitution. The most common mechanisms are the tetrahedral mechanism and the closely related addition elimination mechanism. An unprecedented intramolecular migration of carbon groups from aluminum to an adjacent vinylic center and its application to the synthesis of stereodefined olefins joseph a.
1989 54 5 998 1000. Introduction the addition elimination route is the most studied one in scheme 1. Concerted nucleophilic substitution at an sp 3 carbon typically bimolecular nucleophilic substitution s n 2 reaction is one of the most fundamental reactions in organic chemistry giving a substitution product with inversion of the configuration. It is encountered in nucleophilic substitution.
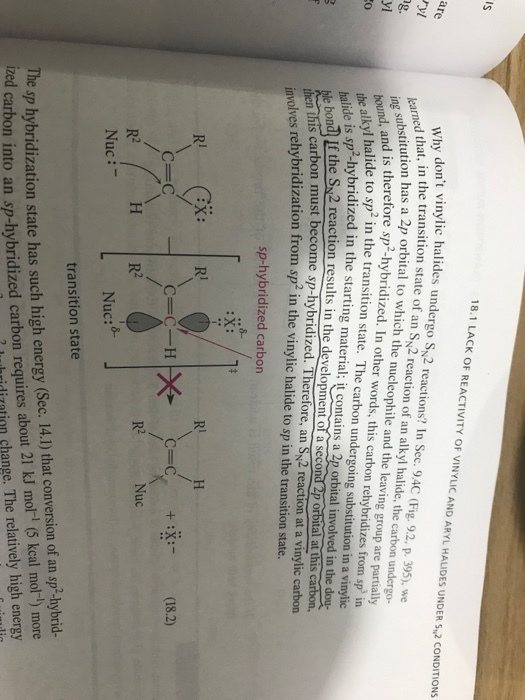
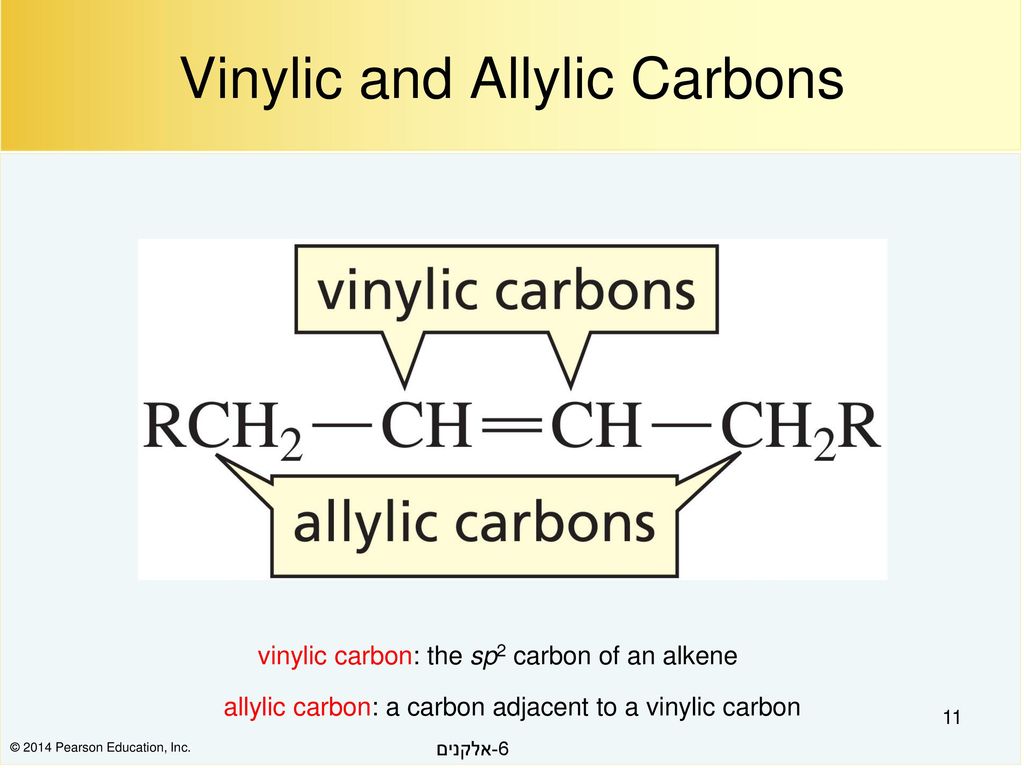
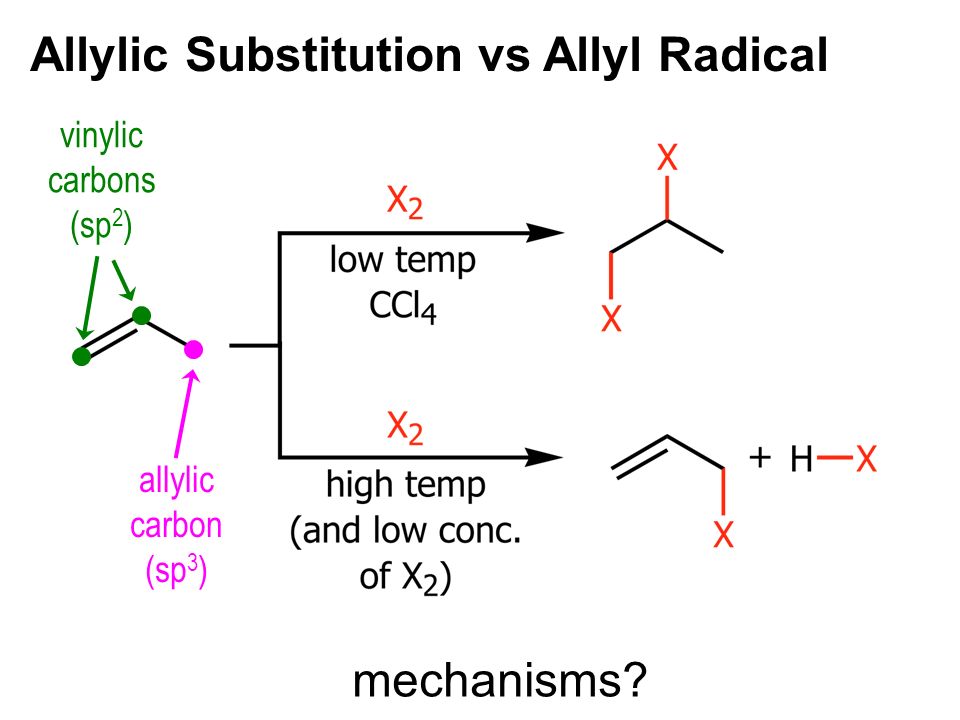
Both of these mechanisms are impossible at a saturated substrate. An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. In this mechanism one bond is broken and one bond is formed synchronously. Nucleophilic substitution at a vinylic carbon 252 is difficult see sec.
10 g i but many examples are known. Chemistry 5 organicchemistry ii 17 nucleophilic substitution at an allylic aliphatic trigonal and s ni reactions and nucleophilic substitution at a vinylic carbon reactivity effects of substrate. This explains the product distribution or. They proposed that there were two main mechanisms at work both of them competing with each other.
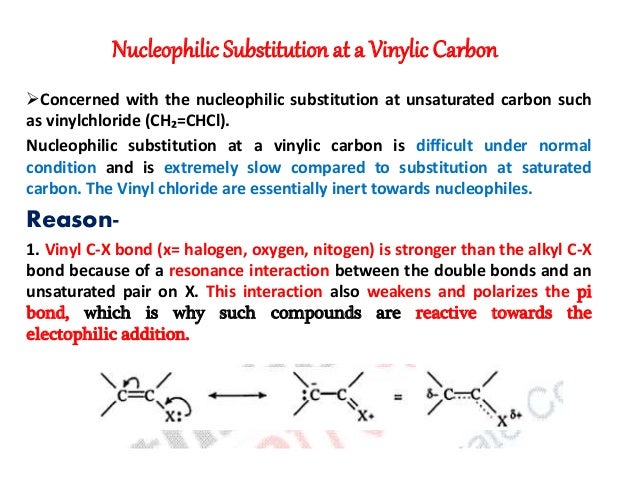
S n2 s n2 is a type of reaction mechanism that is common in organic chemistry. In reaction conditions that favor a s n 1 reaction mechanism the intermediate is a carbocation for which several resonance structures are possible. S stands for chemical substitution n stands for nucleophilic and the number represents. Vinyl c x bond x halogen oxygen nitogen is stronger than the alkyl c x bond because of a resonance interaction.
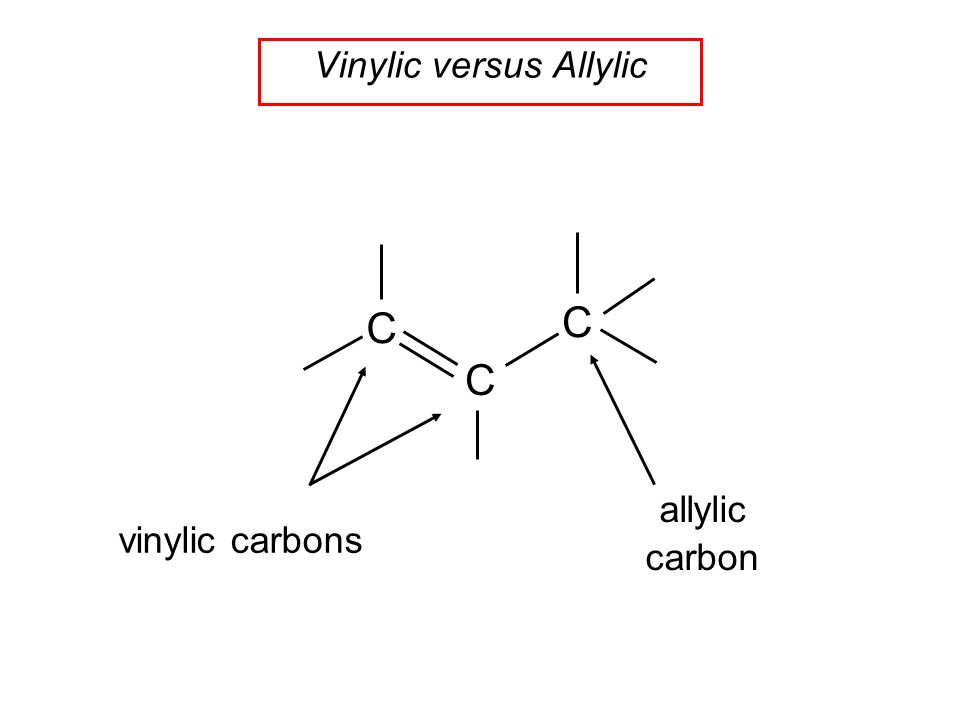
Hughes and sir christopher ingold studied nucleophilic substitution reactions of alkyl halides and related compounds. The vinyl chloride are essentially inert towards nucleophiles. The key difference between allylic and vinylic carbon is that allylic carbon is the carbon. 1 for such a concerted bimolecular nucleophilic substitution at a vinylic sp 2 carbon are proposed two possible mechanisms namely in plane.
Nucleophilic substitution at a vinylic carbon is difficult under normal condition and is extremely slow compared to substitution at saturated carbon. The two main mechanisms are the s n 1 reaction and the s n 2 reaction.